Abstract
Therapy for pediatric acute myeloid leukemia (AML) requires long term central vascular access, which puts patients at risk for central line-associated bloodstream infections and their associated morbidity and mortality. Options for central venous catheter (CVC) access include tunneled external catheters, implanted ports, or peripherally inserted central catheters (PICC). Multiple studies provide conflicting reports on which CVC type is least likely to be associated with bloodstream infections in pediatric hematology-oncology patients. This multicenter cohort of pediatric AML patients is the first to investigate the course-specific incidence of positive CVC blood cultures by line type.
The study included patients receiving frontline chemotherapy for AML between 1/1/2012 and 6/30/2017 at 12 US institutions. Trained personnel completed manual chart abstraction from 12/1/2015 to 07/15/2017 to obtain information on demographics, diagnosis, treatment, antibacterial prophylaxis, CVC type at the start of each course, neutropenia duration, and blood culture results obtained from CVC. Abstractors followed a standardized protocol and were required to achieve >95% concordance with trainer before starting formal chart review.
Course-specific follow-up began the first day absolute neutrophil count (ANC) fell below 200 cells/uL and continued to the earliest of first positive CVC culture (overall and separately for gram-positive and gram-negative species), ANC recovery, start of conditioning for stem cell transplant, relapse, death or start of next course. Per National Healthcare Safety Network guidance, common commensals were not considered true events with the exception of viridans group streptococci (VGS). Nonbacterial pathogens were also excluded. Covariate distributions were compared by CVC type using chi-square tests. Variability in CVC type by site was examined graphically. Incidence rates for CVC positive blood cultures were reported by line type as per 1000 neutropenic days. Multivariate Poisson regression with log of neutropenia duration as offset was used to compute adjusted rate ratios (aIRR) comparing rates of positive CVC blood cultures by line type, controlling for covariates itemized in Table 1. General estimating equation methods were used to account for non-independence of observations from patients from the same institution.
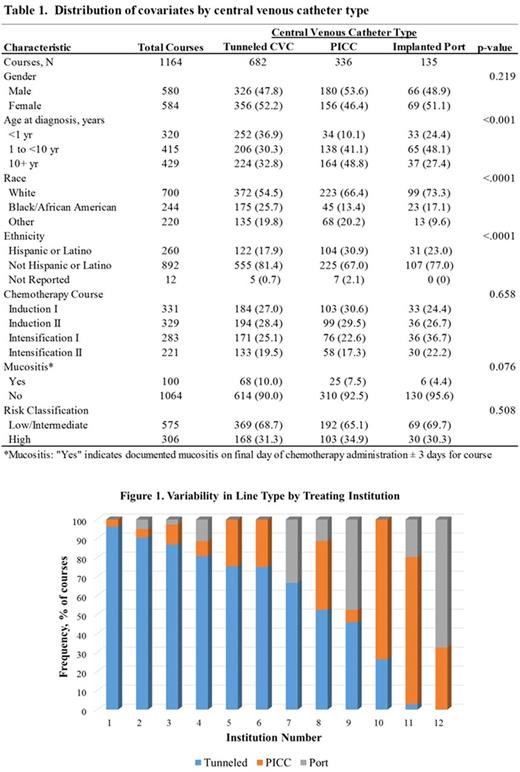
The study population included 357 patients who received 1,164 chemotherapy courses. Median course duration was 38 days (IQR: 34-44) with a median neutropenia duration of 19 days (IQR: 14-25). CVC were present in all patients at the start of each course, with the exception of 11 (3.3%) patients lacking a CVC at the start of Induction I. While tunneled CVC were most prevalent representing 59.1% of courses, PICC and implanted ports were utilized in 28.9% and 11.6% of courses, respectively. There was considerable variation in the distribution of CVC type by treating institution (Figure 1). Age, race, and ethnicity distributions varied by line type (Table 1). Antibacterial prophylaxis was utilized in 47% of courses.
In total, there were 300 (25.8%) courses in 199 (55.7%) patients complicated by a positive CVC blood culture during the neutropenic period. The overall incidence rate of positive CVC blood cultures was 6.5 per 1000 neutropenic days. Rates increased over the course of frontline treatment from 3.1 per 1000 neutropenic days in Induction I to 9.6 per 1000 neutropenic days in Intensification II. The rate of gram-positive events (4.2 per 1000 neutropenic days) was higher than gram-negative events (2.0 per 1000 neutropenic days). The most common isolated organisms were VGS at 57.3% and less than 1% of events were polymicrobial infections. Overall rates of positive CVC blood cultures were higher for PICC relative to tunneled CVC (aIRR=1.19, 95% CI: 1.02, 1.38), while rates for implanted ports and tunneled CVC were similar (aIRR=1.06, 95% CI: 0.75, 1.51).
While bacteremia is an expected complication during post-chemotherapy neutropenia and not always due to the presence of a CVC, the risk for a positive CVC blood culture is significantly increased for children with PICC compared with those with tunneled CVC or implanted port. Also, the risk was higher after Induction I and increased with age. Given the notable practice variation in line choice, these data highlight an opportunity for standardization with a goal to reduce infectious complications.
Fisher: Pfizer: Research Funding; Merck: Research Funding. Gramatges: Bristol Meyer Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal